1-2 Bachillerato (16-18 years old)
In this experience, we will discover which chemical elements a star is made of.
Material that could add value if prepared before coming to ESAC:
-
The concept of spectroscopy: emission and absorption lines
-
Videos
Material to be used at ESAC: Scientific Case and the research material.
Sir Isaac Newton was the first person on explaining why a sunray, that goes through a crystal prism with a particular angle, splits the light into different colours. Following the same reasoning, he assumed that the light, coming from other stars, would also be separated by a prism in a similar way.
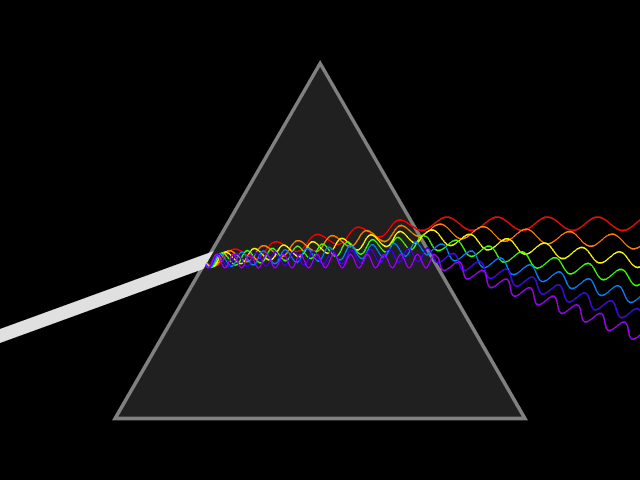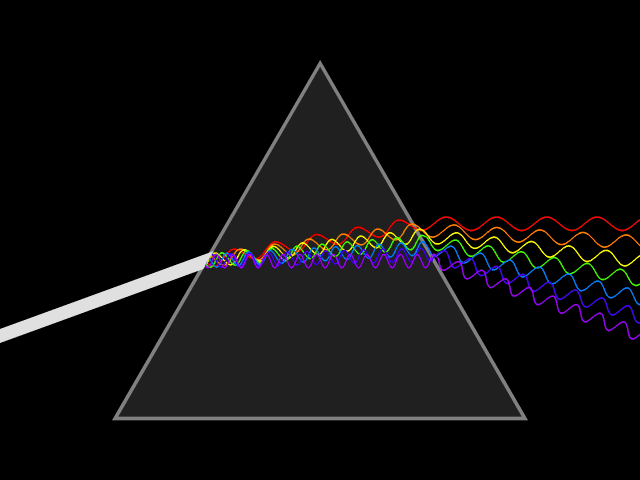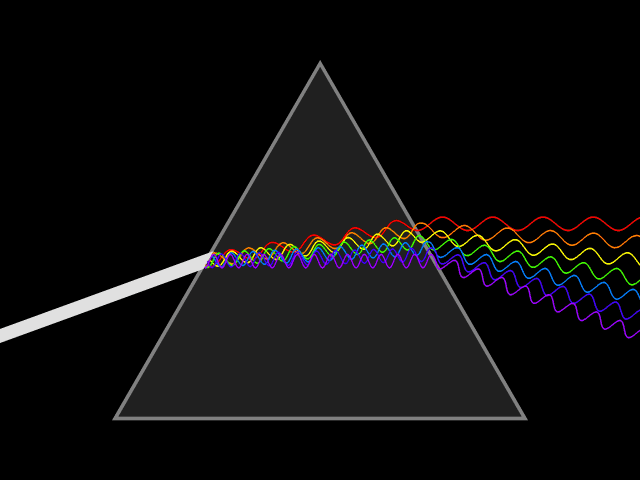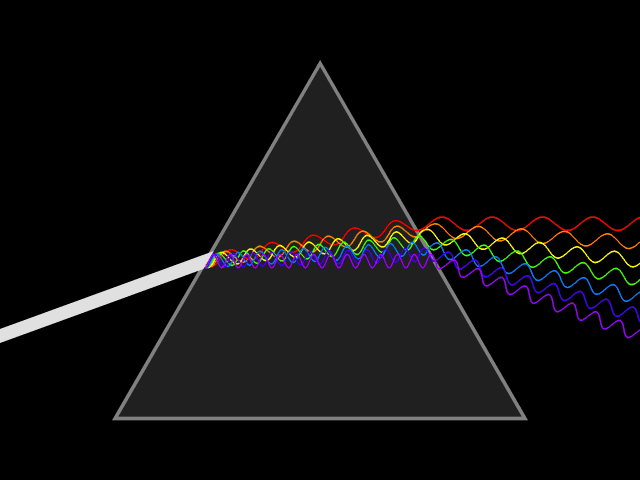
A triangular prism dispersing a beam of white light. The longer wavelengths (red) and the shorter wavelengths (blue) are separated. Credits: Wikipedia
Further and more resolution studies showed up that this spectrum was not continuous but it had several black lines along. Cecilia Payne (1900-1979) discovered that the lines that are emitted by Hydrogen are a match for some of the Sun's absorption lines; thus, there is hydrogen in the Sun !
In other words, if we know the emision spectrum we inmediatelly know the absorption one. If we applie this idea to any star, we will know its composition.
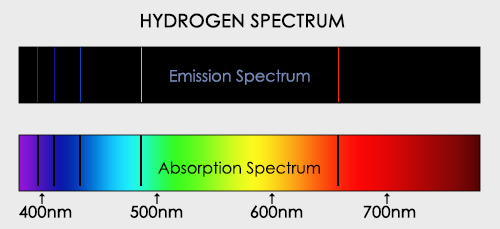
Emmision vs Absorption spectrum. Credit: https://sciencestruck.com/emission-vs-absorption-spectrum
The stellar composition is related to their temperature, and we can classify them according to this. For a start, we can differentiate among seven main stellar classes: type O, type B, type A, type F, type G, type K and type M). The star classification can be further refined, and more classes can be added, by adding after the letter a number from 1 to 9. But we will limit ourselves to this representative sample.
Specifically, the Sun is “type G” star.
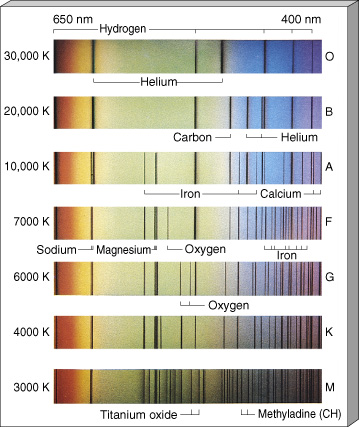
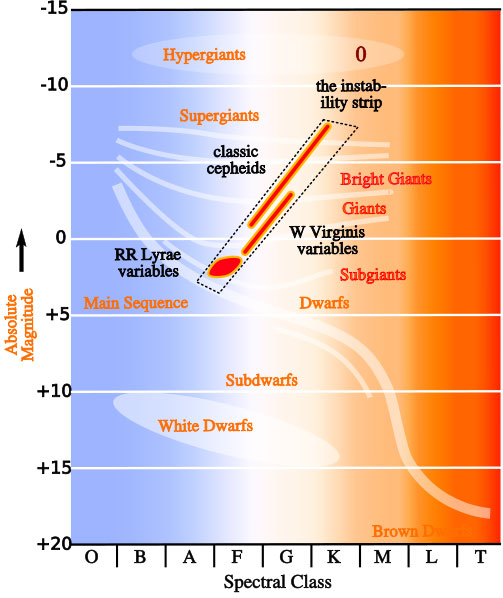
Star Clasification Charts. Credits: ttps://www.e-education.psu.edu and http://www.astro.uchile.cl
The objective of this experience is getting to know the main elements that are inside the Sun and other stars. Would you like to try?